Custom-built GMP Cleanrooms
Patient safety is critical in pharmaceutical manufacturing. This is why our GMP cleanrooms are fully compliant with Good Manufacturing Practice guidelines and the provided User Requirement Specification (URS). If you need an MHRA-compliant manufacturing environment, find out how the experts at Angstrom Technology can help.

GMP cleanroom manufacturers
GMP cleanroom construction principles reduce particulate and cross-contamination risks for pharmaceutical production. Each GMP cleanroom project has oversight from our dedicated Regulatory Governance team. This team has over 30 years’ combined experience in GMP & pharmaceutical industries and will work closely with you on the design, build, and validation processes.
Take a look at the ways we can custom-build your perfect GMP room.

Flush Finish

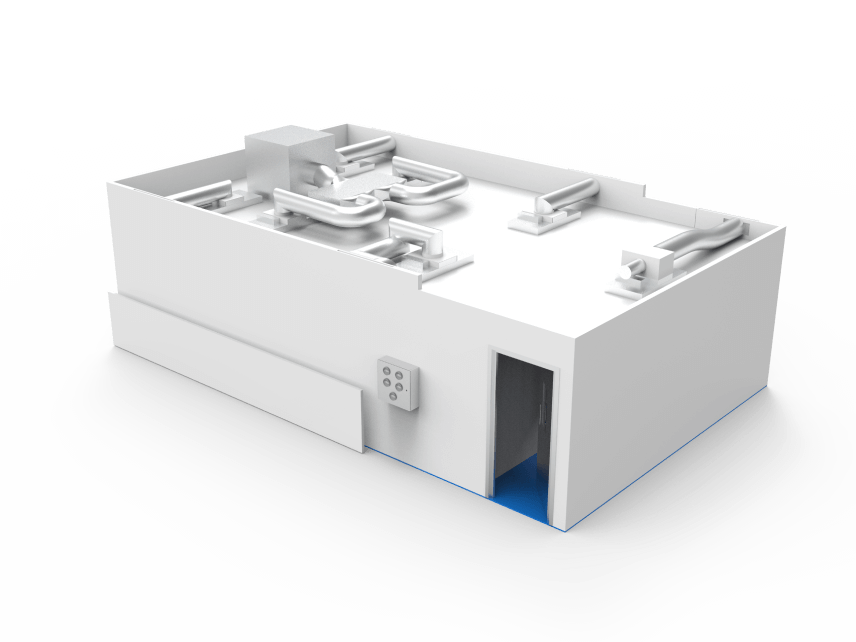
Centralised or decentralised air handling

ECO control system

Material transfer & airlocks

Vision panels
GMP cleanroom qualification
Our GMP cleanroom qualification process meets EU GMP Annex 15 requirements. It covers Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and where necessary Performance Qualification (PQ).
Design Qualification will demonstrate and document the compliance of the design of your facility with GMP and your URS. We will integrate current applicable regulatory requirements — including the newly released EU GMP Annex 1 — and guarantee the performance of the GMP rooms based on the parameters laid out in your URS.
We will work closely with you to make sure you have everything you need for a successful inspection.

Installation Qualification
Installation Qualification is a documented process that ensures the facility and equipment provide exactly what is required.

Operational Qualification
Operational Qualification verifies that the installed facility and associated equipment operate in accordance with the design specifications.

Performance Qualification
Performance Qualification verifies that the facility and associated equipment operate as intended under load in accordance with the design specification in a worst case scenario.
Meet our regulatory governance team
 Joan Benson
Joan Benson
Global compliance and quality assurance manager
Joan has 30 years previous experience in Cell and Gene Therapy, Academia, Pharmaceutical Industry, Contract Clinical Research and Hospital Aseptic facilities. She was front facing regulatory inspections in MHRA, FDA, HTA and HFEA for 20 years.
Simon Rice
Global compliance lead
Simon has 8 years previous experience in the production of radiopharmaceuticals within a fully accredited GMP facility manufacturing sterile pharmaceuticals. This gives him a unique understanding on the implications of GMP.

Download the regulatory governance team brochure
All cleanroom projects with a requirement for GMP will have oversight from our dedicated regulatory governance team. With over 30 years’ combined experience in GMP & pharmaceutical industries, this level of in-house expertise sets us apart from other cleanroom companies.
We also provide a GMP consultancy service including audits of existing facilities and Quality Management Systems including pre-inspection or mock audits in readiness for a regulatory visit or a customer audit.
GMP cleanroom design & build projects
Our complete systems are qualified to EU GMP or international cGMP, and also the required ISO classification. Not only that, but they can also meet any applicable international engineering and building standards and regulations.
We’ve developed proven envelope solutions and reliable cleanroom HVAC designs for many applications. As part of the Angstrom Technology group of companies, we pride ourselves on our in-house expertise and our capability to deliver over 100 cleanrooms every year across the UK, Europe and America. Including:
- 33-room manufacturing facility with 5 cleanrooms in Edinburgh for cell & gene therapy manufacture, and incorporates a training academy
- 80m² cleanroom in Cardiff for the aseptic filling of intravenous drugs
- 6,000m² cleanroom in Cardiff for healthcare production

BioCube cell & gene therapy suite for RoslinCT - GMP Grades B, C & D
Industry: Healthcare | Pharmaceutical
Wall type: Monobloc – fully flush
Classification: GMP grade B / C / D

A Sterile Manufacturing Cleanroom Facility for the NHS - GMP Grades B, C & D
Industry: Healthcare
Wall type: Monobloc – fully flush
Classification: GMP grade B / C / D

Cytiva supports global biopharma industry with 6,000m² cleanrooms in Cardiff, Wales
Industry: Healthcare
Wall type: Monobloc – fully flush
Classification: ISO 7

START A PROJECT WITH US
Our design and build specialists have experience working with customers in all kinds of industries on a global scale, achieving great results time and time again. We’d love to work with you as well!
REQUEST A QUOTE
