A Sterile Manufacturing Cleanroom Facility for the NHS - GMP Grades B, C & D
NHS Wales partnered with Angstrom Technology to facilitate in the creation of a purpose-built cleanroom facility, including GMP qualification from DQ-PQ.

Key facts
Fast-tracked GMP cleanroom
Ready for MHRA inspection in just 6 months
80m² cleanroom
5 zones of GMP Grades B, C & D
Safe transfer of materials & people
Through interlocking doors and transfer hatches
ECO2 monitoring and control system
21 CFR 11 compliant audit trail for traceability and protection of data
What did our client need?
In March 2020 during the first COVID-19 surge, the UK Government increased Critical Care Unit (CCU) bed numbers by 3-4 times. However, it wasn’t possible to increase the number of critical care nurses overnight. With an urgent requirement for the nurses who have this specialist training to cover more beds than they would normally do, it explored ways in which CCUs could apply lean principles to generate efficiencies.
One of the main activities that takes critical care nurses away from patients is making up their intravenous drugs. The Welsh Government decided to create a central manufacturing capacity to make the sterile injections for critical care to support CCUs right across Wales.
The MHRA determined that this process would need to be conducted in cleanroom facilities, so NHS Wales partnered with Angstrom Technology. It fast-tracked a purpose-built cleanroom facility, including GMP qualification from DQ-PQ. It was ready for MHRA inspection in just 6 months.

How did we help our client?
Angstrom Technology expedited the design and build of a temporary medicines unit for NHS Wales’ new Central Intravenous Additive Service (CIVAS). The new CIVAS temporary medicines unit can produce up to 2600 syringes per week, to meet the forecasted demand during the second winter wave.

How did our client benefit?
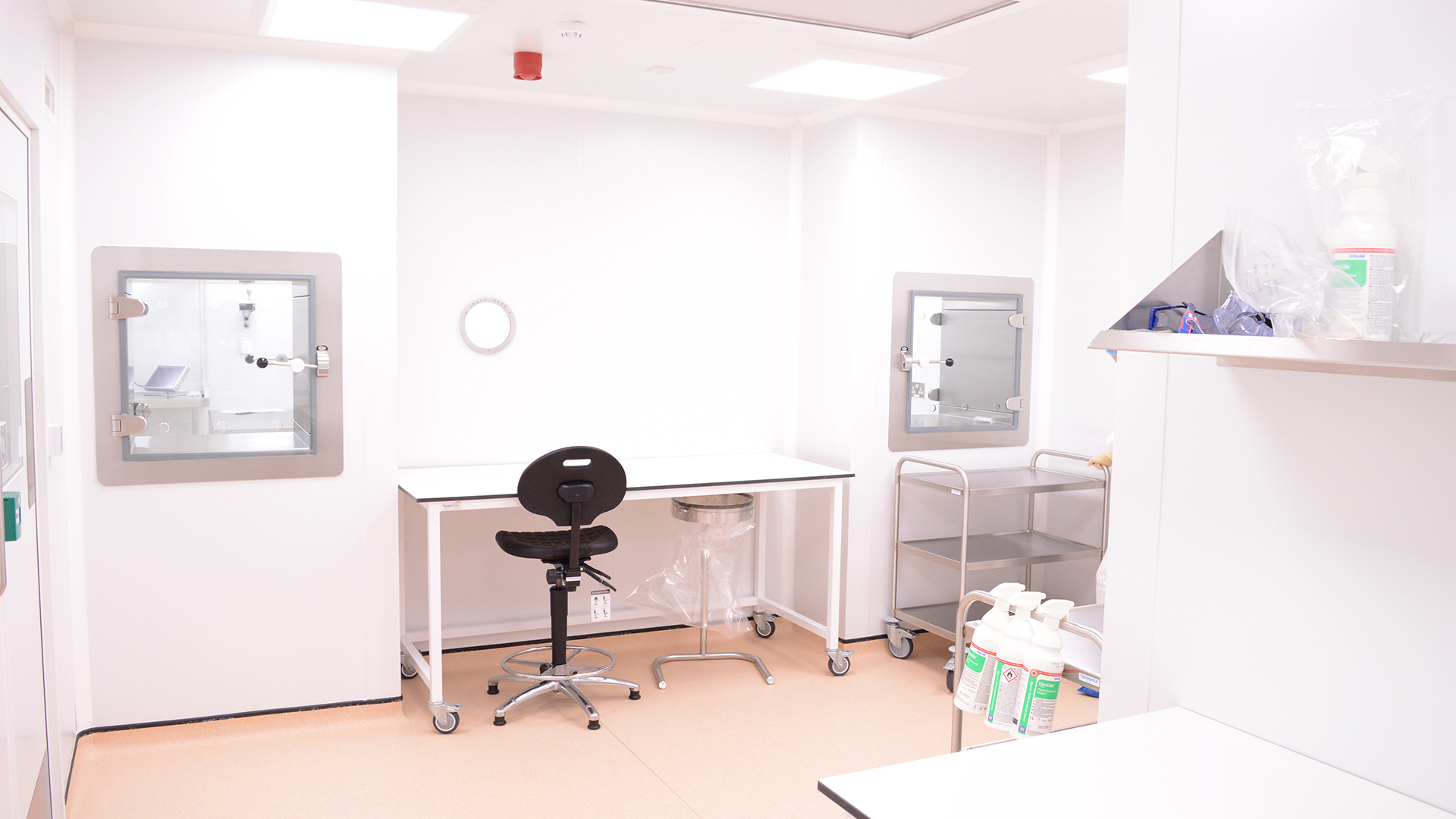
The 80m2 cleanroom features five zones of GMP Grades B, C & D. An UltraTech panel system creates a flush finish to walls and ceilings with a minimum of projecting ledges.
Integrated doors feature an interlocking system to create airlocks that protect the integrity of each zone as staff move through the facility. Safe transfer of materials in and out of the facility is provided through diffusion pathway interlocking transfer hatches, that maintain pressure cascades and airflow management.
Angstrom’s ECO2 integrated monitoring and control system gives real-time visibility on the cleanroom’s performance by monitoring pressure differentials, temperature, relative humidity, particle counts and other requirements. NHS Wales can record batch ID against datasets and the 21 CFR 11 system meets audit trail requirements for traceability and protection of data.


Angstrom Technology recognised the importance of the project and it’s been completed amazingly quickly. The company pulled out all the stops to begin with at design stage and the same urgency went right the way through the construction phase.

START A PROJECT WITH US
Our design and build specialists have experience working with customers in all kinds of industries on a global scale, achieving great results time and time again. We’d love to work with you as well!
REQUEST A QUOTE