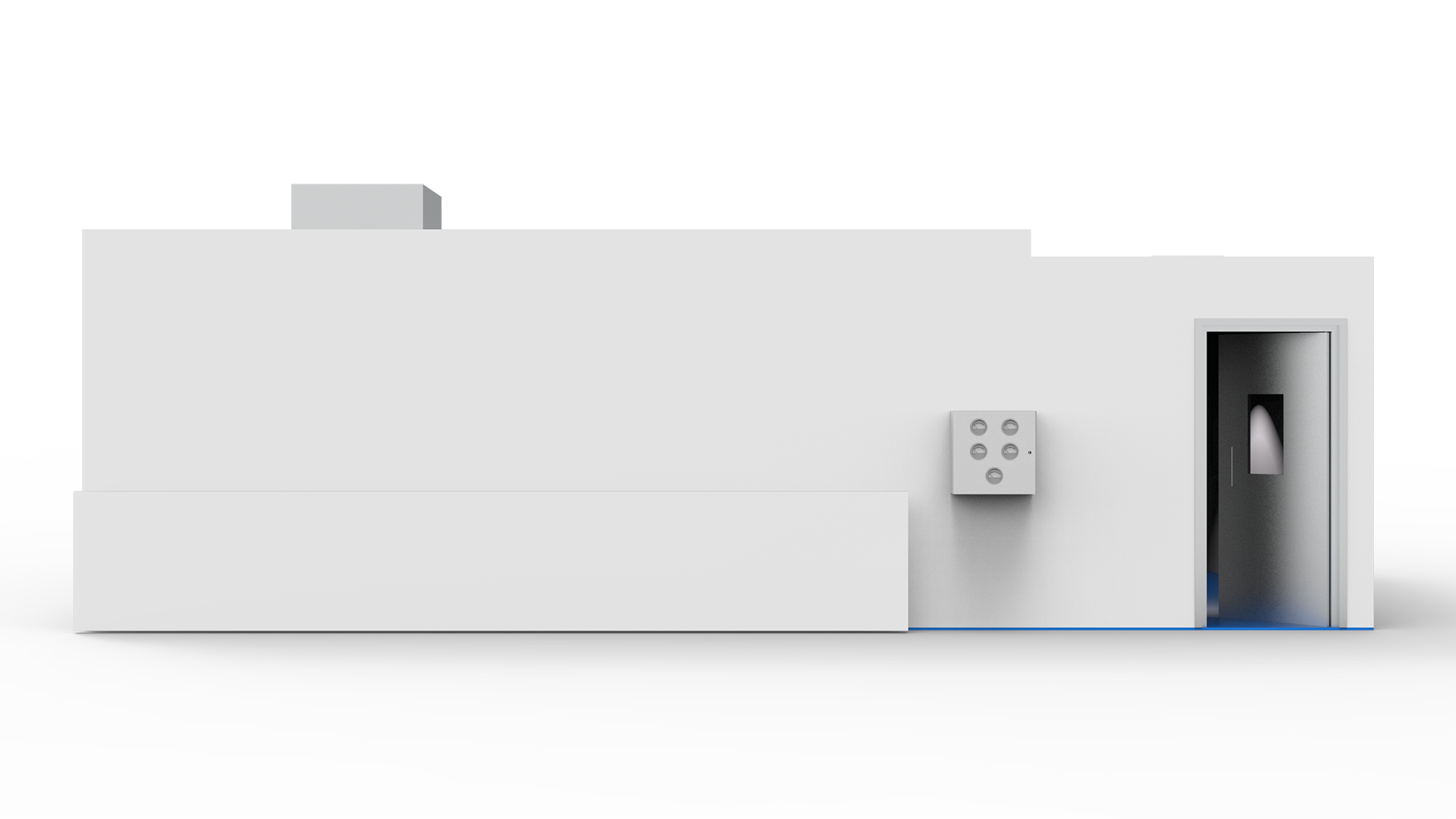
Monobloc Cleanroom for Solid and Liquid Dose Medicine Production
Cubic Pharmaceuticals required a cleanroom suite, for the manufacture of solid and liquid dose medicines.
Key facts
54m²
GMP grade cleanroom
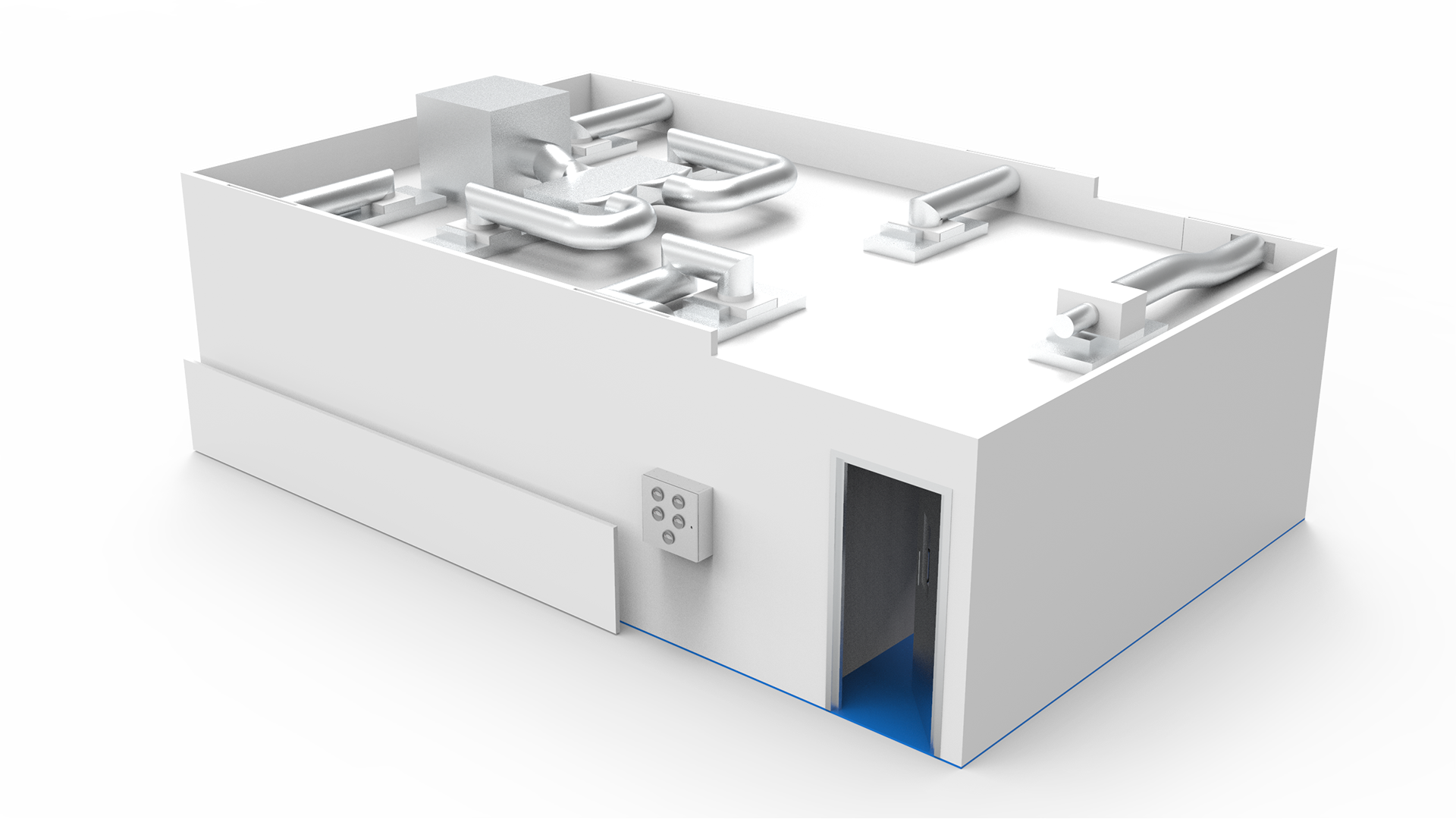
5 areas for mixing, production, cleaning and dispensing
Off-site constructed for an efficient installation
What did our client need?
Cubic Pharmaceuticals required a cleanroom suite, for the manufacture of solid and liquid dose medicines. Cubic Pharmaceuticals is the first life science company in the UK to achieve a patented design for its novel continuous granulation process for processing water insoluble Active Pharmaceutical Ingredients (APIs) by means of hot melt extrusion (HME).
With a unique continuous manufacturing process for processing most challenging APIs, its technology increases the solubility of ibuprofen to 80% within 15-20 minutes and 97% within 2 hours.
“We invented the manufacture of Ibuprofen through Hot Melt Extrusion, which is a world first”, says Saumil Bhatt, Cubic Pharmaceuticals. “We also use platform technology to manufacture another 30 different non-soluble molecules through Hot Dry Extruded Granulation.”

How did we help our client?
The monobloc cleanroom suite encompasses 5 areas for mixing, production, cleaning and dispensing, plus a change area for operatives. It was off-site constructed for an efficient installation.

How did our client benefit?
“This GMP grade cleanroom has transformed Cubic Pharmaceuticals to a Contract Research Organisation (CRO), allowing pharma companies to benefit from our technology breakthroughs,” says Saumil Bhatt, Cubic Pharmaceuticals.


This GMP grade cleanroom has transformed us to a Contract Research Organisation (CRO), allowing pharma companies to benefit from our technology breakthroughs.
We used C2C because of the amount of projects they have delivered, the affordable cost and their track record of high quality work.

START A PROJECT WITH US
Our design and build specialists have experience working with customers in all kinds of industries on a global scale, achieving great results time and time again. We’d love to work with you as well!
REQUEST A QUOTE