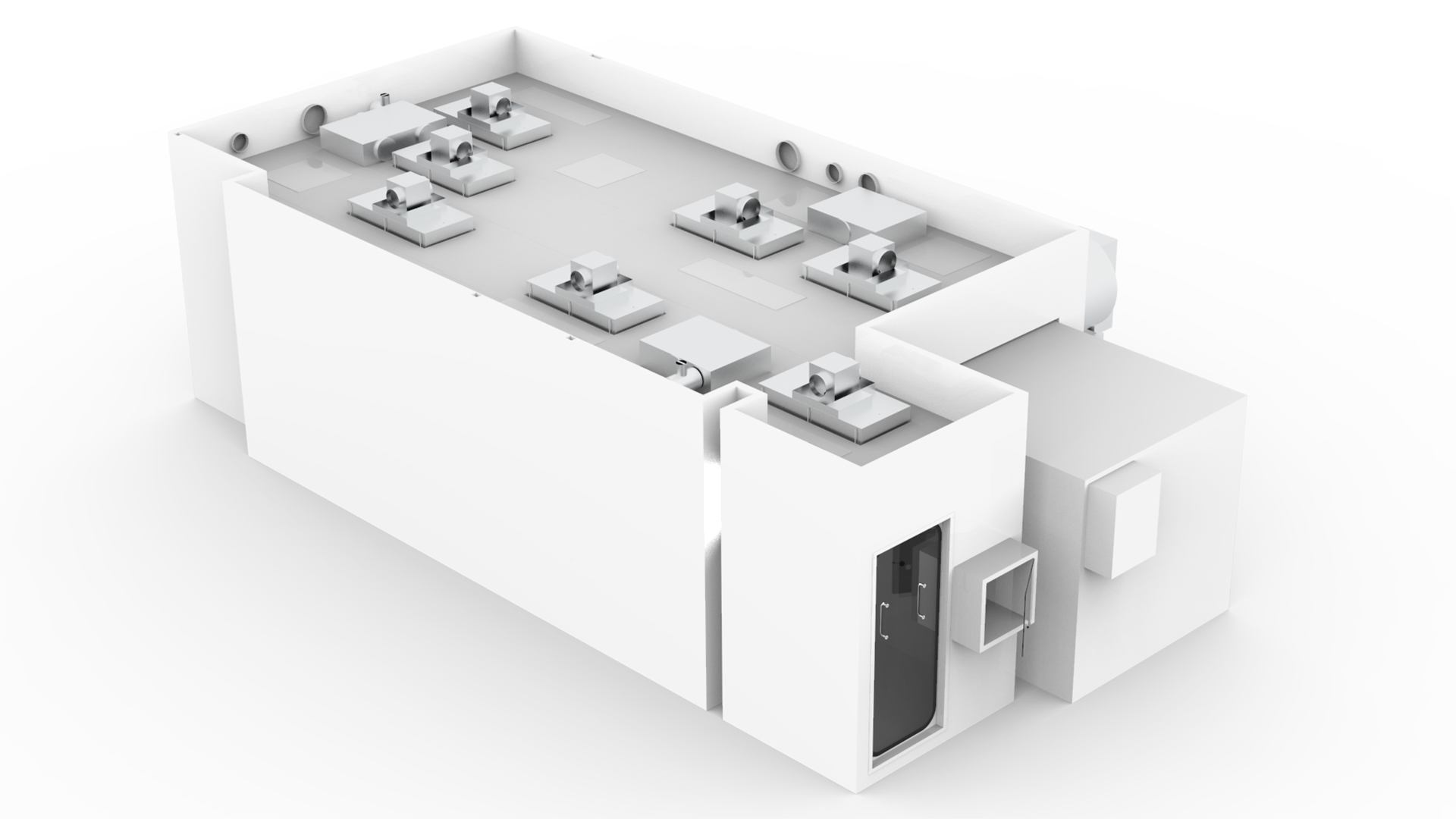
Cleanroom facility for the production of medical collagen products
CambCol’s custom-built ISO Class 7 monobloc precision panel cleanroom was delivered for the production of its range of high quality, medical collagen products to provide benefits across a range of applications.
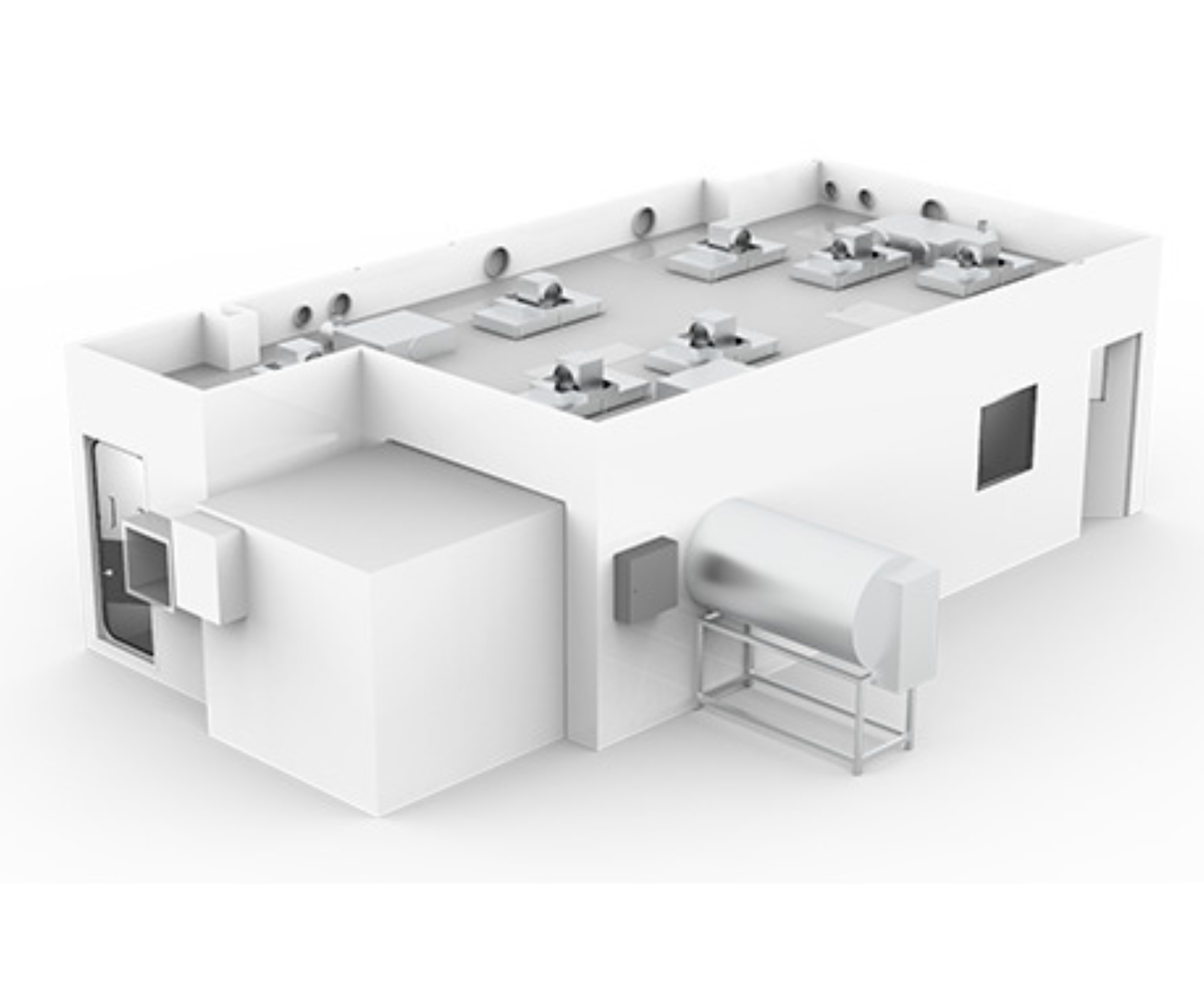
Key features of the cleanroom
50m² cleanroom facility
For the production of medical collagen products
ISO class 7
Adhered to GMP design principles
Process-built layout
Designed using digital modelling
Part-enclosure of machinery
To control heat gains
Gallery
The client
CambCol is a Cambridgeshire-based company that has developed a range of high quality, medical collagen products to provide benefits across a range of applications.
As CambCol are producing a medical-grade product, the specification of the ISO cleanroom adhered to GMP design principles. With its UltraTech Precision panel system’s fully flush envelope, the facility is futureproofed to meet emerging regulations.
The project
Using our reliable design process, we used digital modelling to calculate and verify the air flow design, reducing risk and enhancing performance. HEPA fan filter units (FFUs) were used to process and deliver the particulate control and the air change rate (ACR) required to achieve ISO 14644 Class 7.
Our ECO control system gives real-time visibility on the cleanroom’s performance and alarms if any monitored parameters vary outside of a user-defined threshold.

The cleanroom
The cleanroom was designed in zones to facilitate CambCol’s process and equipment for milling and freezing. Some machinery was part enclosed, to control the heat gains within the classified areas and doors and vision panels were incorporated to facilitate process flow.
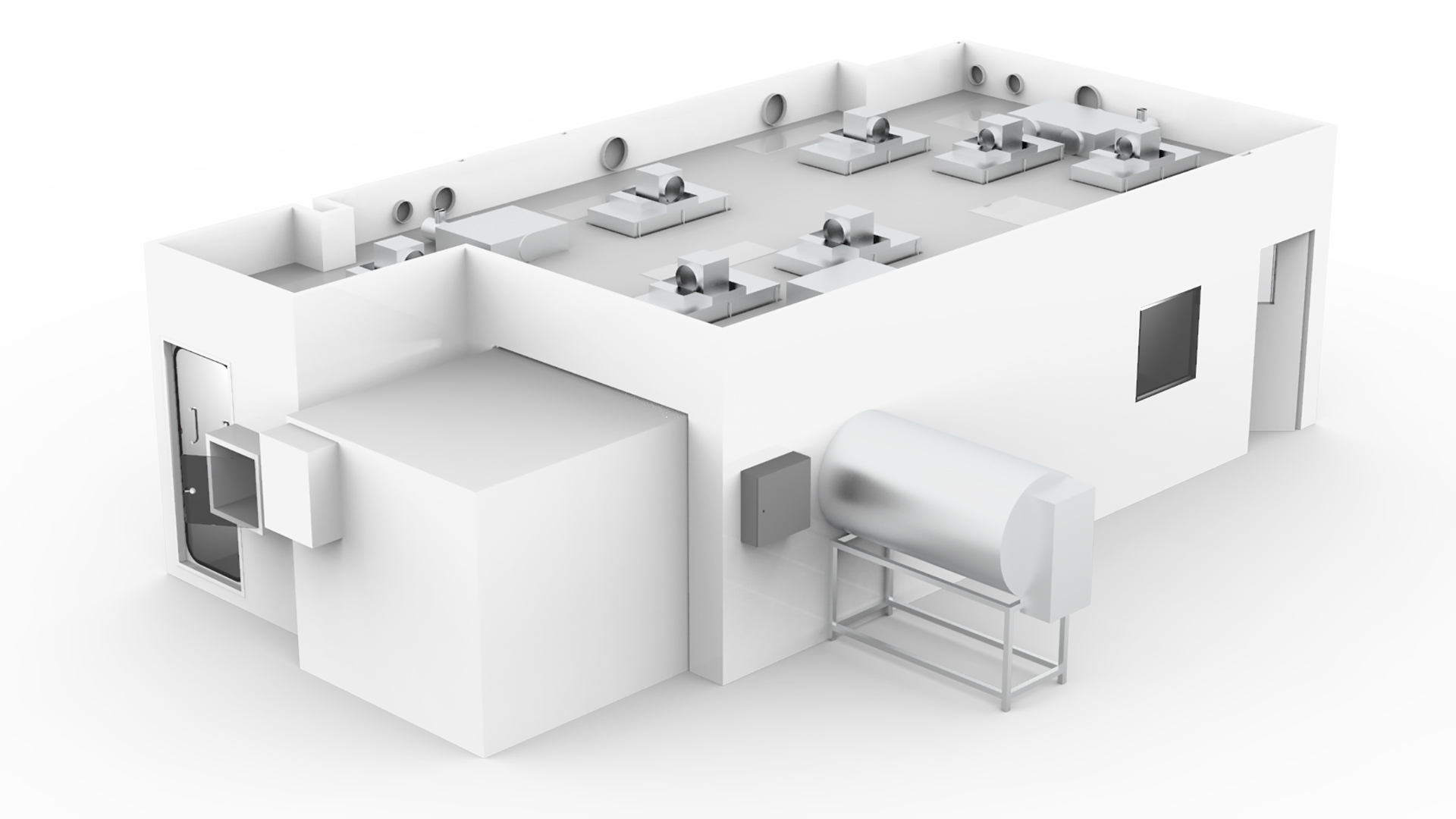
We provided a full furniture fit-out service, coordinated with the cleanroom installation to reduce overall project lead times and benefitting CambCol with the opportunity to use a single point of supply.
Cleanroom validation was performed to qualify performance in accordance with ISO 14644, with an interim report issued immediately and the full report to meet the needs of regulatory bodies issued in just 2 days.


Start a project with us
Our design and build specialists have experience working with customers in all kinds of industries on a global scale, achieving great results time and time again. We’d love to work with you as well!